Want to join the fight against cancer?
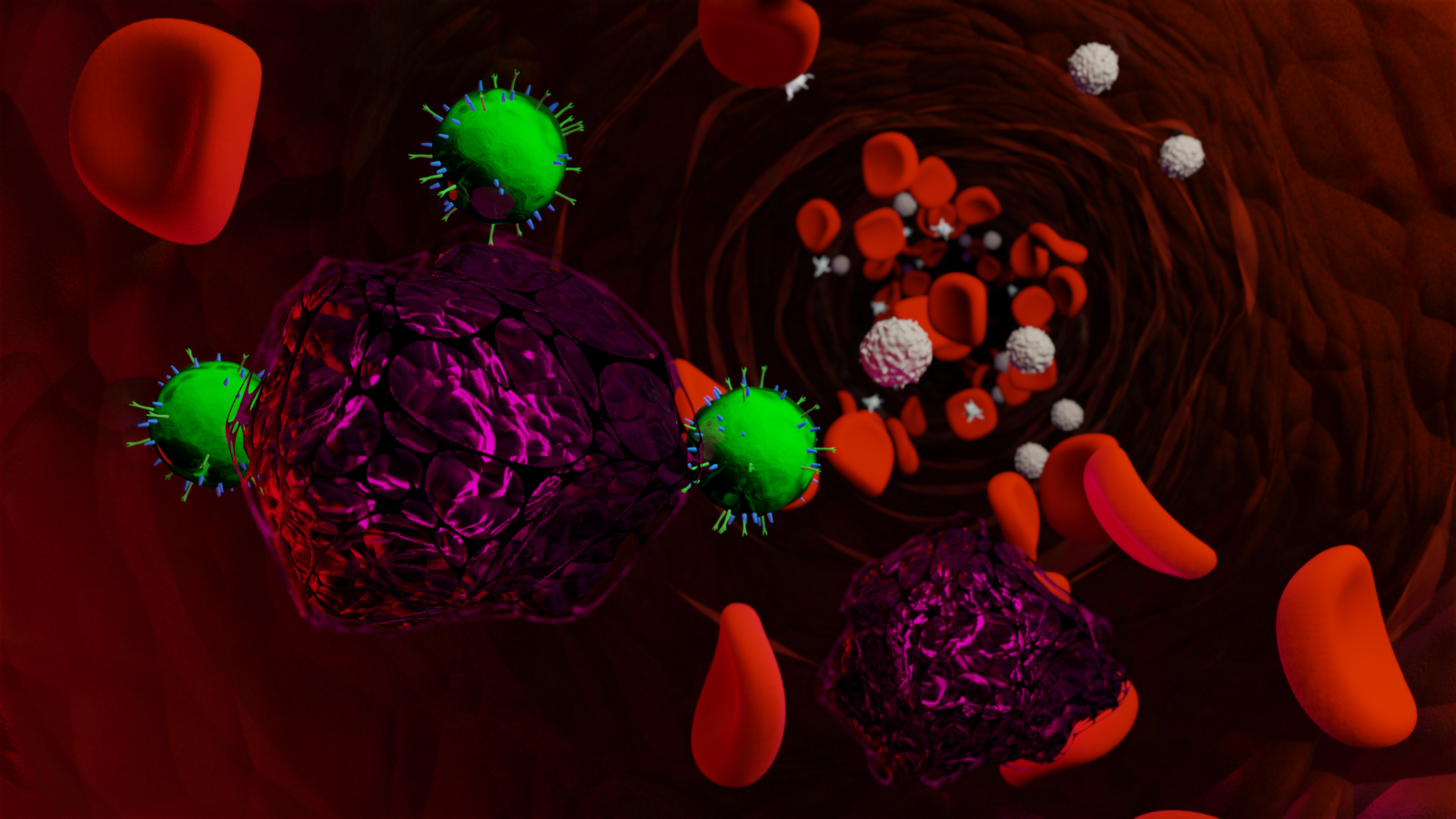
Exhibit Details
Open FebNov 2021
Level 1Futures Gallery
- The Brief
- Let's Complicate Things
Could the answer to treating cancer be found in your own blood?
Upstairs in this very building, researchers are investigating CAR-T therapy. This new treatment hacks your immune system to turn it into a cancer killer.
Enter the blood stream, Magic School Bus style, and check it out from the inside.
Delve Deeper
There’s no single cure for cancer, because each cancer is a complex system.
Cancer is out-of-control growth of cells in the body. But it’s not disorganised. Rather than growing as a random blob, cancer cells are able to self-organise in ways that help them grow, for example by blocking out immune cells or making their own blood vessels to feed themselves.
Find out why cancer is linked to self-organisation here.
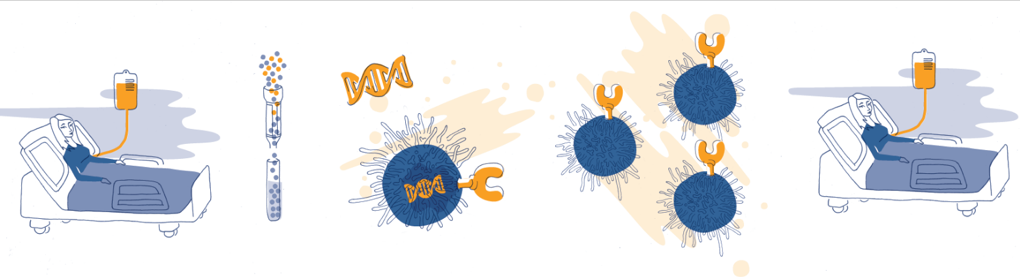
Chimeric antigen receptor T cell (CAR-T) therapy harnesses the body’s immune system to fight cancer.
CAR-T cells are made from a patient’s own T cells, key cells of the immune system. The T cells are isolated from a sample of the patient’s blood and then genetically modified to express a chimeric antigen receptor (CAR) on their surface. The CAR is targeted to recognise a specific marker on the surface of cancer cells.
Cancer is complex
Cancer is a complex system, where lots of elements interact in a non-linear and unpredictable manner. A tumour can be viewed as an emergent, self-organising property of a complex system within the body.
T-cells are killers
T-cells are a vital component of our immune system that can detect and kill abnormal cells that might be infected or cancerous. Cancers can only grow once they escape detection of the immune system by modifying their surface proteins.
Hacking your own immune system
CAR-T therapies start with taking the patients own blood and genetically engineering their T cells so they can recognise, target and kill the cancer. See this video for a good summary.
Cancer cells can be hard to get to
CAR-T therapies have been developed and approved for blood cancers like leukaemia where it’s easier for the injected cells to find and destroy the cancer cells, which are also circulating in the blood.
It gets harder when dealing with a solid tumour – it’s difficult to get out of the blood vessels to where the cancer is, and it’s difficult for the CAR-T cells to get to the cells in the middle of these tumours. Carina Biotech are developing CAR-T cell therapies especially for solid tumours.
Side effects – cytokine storms and suicide switches
In CAR-T therapies, toxicity and efficacy go hand in hand. If it’s working, there will be side effects. If the immune system goes into overdrive it can result in a complex cascade of events known as a ‘cytokine storm’ which can (and has) led to fatally adverse events in some trials.
Once CAR-T cells have been delivered, there’s no way to get them out again. As a living drug, it’s not like a normal medicine that will eventually be metabolised and leave the body, once they’re in – they’re in! So if something goes wrong researchers are now engineering CAR-T cells to have a ‘suicide switch’ – a way of inducing the cells to die off with the addition of a small molecule drug.
Ethical considerations for CAR-T cell therapies
- Safety concerns (see side effects above). In clinical trials for the two currently available treatments, all patients reported adverse side effects with 84-95% experiencing severe side effects (including six deaths across both trials). While these deaths were mostly attributable to ‘cytokine storms’, there are other severe neurological side effects which are significant and still not well understood.
- Managing expectations and hype. There is a lot of excitement about the possibilities of CAR-T therapies, but still uncertainty about the long-term benefits and risks, like how long patients will stay in remission. You don’t want to see what has happened in the stem cell industry – where a growing number of unproven therapies are aggressively marketed to vulnerable patients. Currently the treatment is one of last resort.
- Equitable access both financially and geographically. CAR-T therapies are expensive. CAR-T cell therapy treatment in Australia was only approved for use by the Therapeutic Goods Administration in December 2018, at a cost of $598,000 per patient. In the US Novartis, which produces Kymriah to treat paediatric leukaemia, offer the drug in an unusual pay-for-performance model where patients who do not respond within the first month after treatment do not have to pay. This is also just the cost for the therapy, not the management of expected adverse events. Even at the clinical trial level, the demand for slots in trials far exceeds capacity, which runs the risk of privileging some groups over others.
Discover more
Watch:
- UniSA’s fight against cancer – Dr Tessa Gargett
- How to biohack your cells to fight cancer – Greg Foot (TEDed)
Read:
- UniSA Cancer Research
- New approach to fighting cancer – UniSA
- The Science behind CAR-T Therapy – Carina Biotech
Listen:
Find this exhibit at the UniSA Library
Credits
- Arterial Design Design and Build
- Guillermo Gomez Research, Centre for Cancer Biology
- Jade Feong Research, Carina Biotech
- Justin Coombs Research, Carina Biotech
- Lih Tan Research, Centre for Cancer Biology